In this article we will discuss about Riccia. After reading this article we will learn about:- 1. Systematic Position of Riccia 2. Distribution and Habitat of Riccia 3. Reproduction 4. Fertilization.
Contents
Systematic Position of Riccia:
Distribution and Habitat of Riccia:
Riccia, the most widely distributed genus of family Ricciaceae, is represented by about 200 species (Reimer, 1954). The name Riccia was given in honour of P. F. Ricci, a Florentine politician. Widely distributed in both tropical and temperate regions of the world, this genus is represented in India by about 33 species (Puri, 1973).
However, Srivastava (1964) recorded 29 species from different parts of India. All species are terrestrial and prefer to grow on moist and shady places except Riccia fluitans, which is an aquatic species and occurs floating in still stagnant water or submerged below the surface of standing water.
Some of the common terrestrial Indian species are:
R. gangetica, R. discolor, (R. himalayensis), R. glauca, R. crystalline, R. frostii, R. hirsuta and R. melanospora. Riccia gangetica, R. kashyapii and R. pandei are endemic species i.e., confined to Indian territory only.
Gametophytic Phase:
The plant body of Riccia is gametophytic and gametophytes are fleshy, prostrate and dichotomously branched. Repeated dichotomy results into a typically rosette like appearance (Figs. 1, 3).
In Riccia cruciata only two dichotomy result in a cruciate form (Fig. 4). Each branch of the thallus is linear, wedge-shaped or obcordate. Thallus is 5-7 mm long and 1.2 mm broad in all the terrestrial species. However, in R. fluitans it is 30-50 mm long and 1-2 mm broad.
Dorsal Surface:
The dorsal surface is light green or dark green body, each branch having a thick midrib. It is traversed by a conspicuous median longitudinal groove which ends in a depression at the apical region forming an apical notch.
Growing point is situated in the apical notch. The main function of the mid-dorsal groove is to retain water required for fertilization. Some hairy epidermal outgrowths are also seen in Riccia melanospora, though rarely (Fig. 5).
Vental Surface:
The ventral surface of thallus bears many scales and rhizoids. Scales are violet coloured, multicellular and one celled thick structures (Fig. 6). The colour of the scale is due to dissolution of the pigment in the cell sap. Scales are arranged all along the margin in a single row. In the apical region they project forward and
In hygrophilous species (species which need a large supply of moisture for their growth) the scales are ephemeral (i.e., short lived) but in xerophilous species the scales are leafy and persistent. In Riccia crystallina the scales are inconspicuous and absent. Rhizoids are unicellular and un-branched. They develop as prolongations of the lower epidermal cells.
They are of two types:
(i) Smooth-walled rhizoids
(ii) Tuberculate rhizoids.
In smooth-walled rhizoids both the inner and outer wall layers are fully stretched while in tuberculated rhizoids the inner wall layer modifies into peg-like or plate-like in growth which projects into the cell lumen (Fig. 7). The main function of rhizoids is to anchor the thallus on the substratum and to absorb water and mineral nutrients from the soil.
In Riccia fluitans, the only aquatic species, thallus is long, narrow, ribbon-like and dichotomously branched. Rhizoids and scales are absent (Fig. 8). This species can also grow on soil. In terrestrial species thallus bears many rhizoids and small, colourless or violet scales near the apex (Fig. 9).
Anatomy of the Gametophyte:
A vertical cross section of the thallus shows two distinct zones, viz., upper photosynthetic zone and lower storage zone (Fig. 10A, B).
Upper Photosynthetic Zone:
It is green, dorsal on upper region of the thallus. It is made of somewhat vertical rows of un-branched photosynthetic filaments. All the cells of the photosynthetic filament except the uppermost one are isodiametric and possess many discoid chloroplasts. The terminal cells of photosynthetic filaments are large, hyaline and form loose, ill-defined and discontinuous one celled thick epidermis.
Photosynthetic filaments are separated from each other by narrow longitudinal vertical canals called air chambers. Each air chamber is bounded by four epidermal cells (e.g., Riccia glauca, (Fig. 11) or eight epidermal cells (e.g., R. vesiculosa). Each air chamber opens on the dorsal surface by an air pore. Air chambers spaces help in the gaseous exchange.
In aquatic form of Riccia fluitans epidermis is continuous and air chambers are almost completely closed. However, in the terrestrial form of this species each air chamber opens on the upper surface by a small opening (Fig. 12).
In spongy thallus of Riccia crystallina the assimilatory or photosynthetic cells form a loosely- arranged network enclosing large air spaces.
Lower Storage Zone:
This zone represents the ventral tissue of the thallus and lies below the photosynthetic zone. It consists of compactly arranged parenchymatous cells. These cells are devoid of chlorophyll and contain starch as reserve food material. The lowermost cell layer of this zone forms the lower epidermis. Some cells of the lower epidermis extend to form the scales and both types of rhizoids.
Reproduction in Riccia:
Riccia reproduces by vegetative and sexual methods.
Vegetative Reproduction in Riccia:
Vegetative reproduction in Riccia is quite common and takes place by the following methods:
1. Death and decay of the older portion of the thallus:
The thallus in Riccia is dichotomously branched and the growing point is situated in its apical notch. The basal or the posterior part of the thallus starts rotting or disintegrating due to ageing or drought.
When this process of disintegration or decay reaches up to the place of dichotomy, the lobes of the thallus get separated. Thus detached lobes develop into independent plants by apical growth. It is the most common method of vegetative reproduction in Riccia.
2. By adventitious branches:
The adventitious branches develop from the ventral surface of the thallus in species like Riccia fluitans. On being detained, these branches develop into new thalli (Fig. 13).
3. By persistent apices:
Due to prolonged dry summer or towards the end of growing season the whole thallus in some species (e.g., Riccia discolor) dries and gets destroyed except the growing point. Later, it grows deep into the soil and becomes thick. Under favourable conditions it develops into a new thallus. It is more a method of perennation rather than multiplication.
4. By tubers:
Towards the end of the growing season the apices of the thallus lobes get thickened and form the perennating tubers. These are capable to pass on the unfavorable conditions. On resumption of favourable conditions tubers produce new thalli. Tubers are common in Riccia discolor, R. billardieri, R. perenriis and R. vesicata (Fig. 14).
5. By rhizoids:
The apical part of the young rhizoids divides and re-divides to form a gemma like mass of cells in some species (e.g., Riccia glauca). These cells contain chloroplast and are capable of developing into new thallus.
Sexual Reproduction in Riccia:
Sexual reproduction in Riccia is oogamous. Male reproductive bodies are known as antheridia and female as archegonia.
Some species of Riccia like R. crystallina, R. gangetica, R. billardieri and R. glaul are monoecious or homothallic (i.e., both anheridia and archegonia develop on the same thallus) while other species like R. curtisii, R. perssonii, R. bischoffii, R. frostii, R. discolor are dioecious or heterothallic (i.e., antheridia and archegonial develop on different thalli).
Antheridia and archegonia remain enclosed with in the antheridial and archegonial chambers and develop on the dorsal surface of the thallus (Fig. 2, 3). Sex organs develop in acropetal succession (i.e., mature sex organs are present at the posterior end, the young ones towards the apex of the thallus).
In monoecious species alternate groups of antheridia and archegonia develop at a sufficient distance from the growing point. There is no particular time for the development of sex organs, and therefore one can see all the developmental stages in the different sections of the same thallus.
Antheridium:
Development:
The development of antheridium starts from a superficial antheridial cell, situated on the dorsal surface of the thallus (Fig. 15 A). It is a few (2-3) cells away from the apical cell.
The antheridial initial enlarges in size, becomes papillate and divides first by a transverse division to form an upper outer cell and a lower basal cell (Fig. 15 B). Basal cell remains embedded in the tissue of thallus, undergoes only a little further development and forms the embedded portion of the antheridial stalk.
Outer cell divides by transverse divisions to form a filament of 4 cells. Upper two cells of the 4 celled filament are known as primary antheridial cells and lower two cells are known as primary stalk cells (Fig. 15 C-E). Primary stalk cells form the stalk of the antheridium. Primary antheridial cells divide by two successive vertical divisions at right angle to each other to form two tiers of four cells each (Fig. 15 F, G).
A periclinal division is laid down in both the tiers of four cells and there is the formation of eight outer sterile jacket initials and 8 inner primary androgonial cells (Fig. 15 H, I).
Jacket initials divide by several anticlinal divisions to form a single layer of and theridial jacket. Primary androgonial cells divide be several repeated transverse and vertical divisions resulting in the formation of large number of small cubical androgonial cells (Fig. 15 K-M). The last generation of the androgonial cells is known as androcyte mother cells.
Spermatogenesis:
The process of metamorphosis of androcyte mother cells into antherozoids is called spermatogenesis. It completes within a few minutes. Each androcyte mother cell divides by a diagonal mitotic division to form two triangular cells called androcytes. Both the androcytes remain enclosed in the wall of the androcyte mother cell with one separate wall (Fig. 16 A, B).
Each androcyte has a prominent nucleus and a small extra-nuclear granule called blepharopiast. It lies near the periphery of the protoplast (Fig. 16 C). The androcyte soon looses its triangular shape and becomes somewhat round or oval (Fig. 16 D). Its blepharoplast elongates into a cord and occupies about two-thirds of its part.
Simultaneously the nucleus also becomes crescent shaped, homogeneous and ultimately comes in contact with the blepharoplast.
Two large flagella develop from the conspicuously thickened end of the blepharoplast. A small part of the cytoplasm, which is not utilised in the formation of flagella may remain attached to the posterior end of antherozoid as a small vesicle. Each androcyte thus metamorphosis into an antherozoid (Fig. 16 E-H).
Mature Antheridium:
A mature antheridium has a short stalk and oval shaped body with a flat base and conical apex (Fig. 15 M). Stalk attaches the antheridium to the base of the antheridial chamber. Antheridium is present singly in an antheridial chamber. A single-layered sterile jacket encloses the mass of androcytes which metamorphoses into antherozoids.
Mature Antherozoid:
A mature antherozoid is unicellular, uninucleate, biflagellate and coiled structure. Both flagella resemble morphologically but differ in function. One flagellum serves for propulsion and the other for rotation and for changes in direction (Fig. 15 H).
Dehiscence:
Water helps in the dehiscence of the anheridium. Antheridial chamber, in which an antheridium lies, communicates with the clorsal surface of the thallus by terminal opening. The cell walls form the semifluid content of the antheridium during the metamoiphosis. Mature antherozoids remain free in the semifluid substance in the antheridial cavity.
As water enters in their antheridial chamber, the sterile apical cells of the antheridial jacket enlarge by absorbing water, become softened and ultimately break open. The mature antherozoids along with semifluid mass, come out of the antheridium to the antheridial chamber and then to the dorsal surface of the thallus.
Archegonium:
Development:
The development of the archegonium starts on the dorsal surface of the thallus from a single superficial cell, which acts as an archegonial initial (Fig. 17 A). This initial enlarges, becomes papillate and first divides transversely into a basal cell and an outer cell (Fig. 17 B).
There is no further division in the basal cell and it forms the embedded portion of the archegonium. The entire archegonium develops from the outer cell. This outer cell divides by three successive intersecting walls or periclinal vertical walls resulting in the formation of three peripheral initials and a fourth median cell, the primary axial cell (Fig. 17 C-E).
Each of the three peripheral initials divides by an anticlinal vertical division forming two cells. In this way the primary axial cell gets surrounded by six cells (Fig. 17 J, M). These cells are called jacket initials. Six jacket initials divide transversely into upper neck initials tier and lower venter initial tier (Fig. 17 G).
Neck initials tier divides by repeated transverse divisions to form a tube-like neck. Neck of the archegonium consists of six vertical rows and each row consists six to nine cells. Venter initial tier also divides by repeated transverse divisions to form a single layer of swollen venter.
Simultaneously the primary axial small cell divides transversely and unequally to form small upper primary cover cell and large lower central cell (Fig. 17 F). The central cell divides into an upper primary neck canal cell and a lower venter cell.
Primary neck canal cell divides by a series of transverse divisions to form four neck canal cells. Primary venter cell divides only once and forms a small venter canal cell and a large egg (Fig. 17 J, K). The primary cover cell divides by two vertical divisions at right angle to one another forming four cover cells which form the mouth of the archegonium.
Mature Archegonium:
A mature archegonium is a flask shaped structure. It remains attached to the thallus by a short stalk. It consists of upper elongated slender neck and basal globular portion called venter.
The neck consists of six vertical rows enclosing four neck canal cells. The venter consists of a single layered jacket. Twelve to twenty cells in perimeter enclose a small venter canal cell and large egg. Four cover cells are present at the top of the neck (Fig. 17 L).
Fertilization in Riccia:
Water is essential for fertilization. It enters the antheridial chamber. Apical cells of the antheridial wall get swollen by absorbing water. These cells become softened and finally breakdown to release mass of antherozoids. These antherozoids come up to dorsal surface of the thallus from the antheridial chamber where they swim in the thin film of water and reach the mouth of the neck of the archegonium.
In the mature archegonium the venter canal cell and neck canal cells disintegrate and form a mucilaginous mass. It absorbs water, swells up and comes out of the archegonial mouth by pushing the cover cells apart. This mucilaginous mass consists of chemical substances such as soluble proteins and certain inorganic salts of potassium.
Many antherozoids enter the archegonial neck because of the chemotactic response and reach up to egg. One of the antherozoids penetrates the egg and fertilization is effected (Fig. 18A-C). The fusion of the nuclei of male and female gamete results in the formation of diploid zygote or oospore. Fertilization ends the gametophytic phase.
Sporophytic Phase:
After fertilization the diploid zygote or oospore enlarges until it completely fills the cavity of the venter of the archegonium. A wall is then secreted around the oospore. The act of fertilization also stimulates the division of the wall of the venter. It divides anticlinally and periclinally to form a two-layered calyptra along the developing sporophyte.
Development of Sporophyte:
The zygote divides first by a transverse division to form two almost equal sized cells (Fig. 18 D). The second division is at right angle to the first and results in the formation of four cells.
This represents quadrant stage (Fig. 18 E). The next division is also vertical but it is at right angle to the first. An 8-called stage thus results. It is called octant stage (Fig. 18 D). The cells of the octant divide in all possible planes to form a spherical mass of 20-40 cells (Fig. 18 E-H).
The cells of the peripheral region divide by a periclinal division to form an outer layer of amphithecium and the central mass of cells called endothecium. The cells of the amphithecium divide only by anticlinal division to form a single-layered sterile jacket or capsule wall. The endothelium forms the archesporium. Its cells divide and re-divide to form a mass of sporogenous cells (sporocytes, Fig. 18 I-L).
According to Pagan (1932), some of the spore mother cells in Riccia crystallina fail to produce spores and form abortive nutritive cells called nurse cells. He considered these cells as the fore-runnes of elaters found in higher forms of Marchantiales.
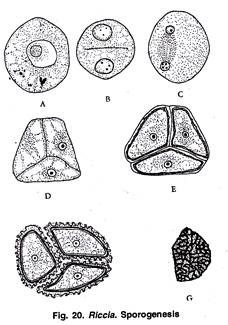
Sporogenesis:
At the time of meiosis, the spore mother cells lie free within the cavity of sporogonium (Fig. 18 L). Spore mother cells undergo meiosis. Nucleus of each spore mother cell divides by two successive divisions to form 4 haploid nuclei.
In first division the chromosome number is reduced to half of the somatic number. The four nuclei migrate to the periphery of the spore mother cell and lie at equal distance from each other. Simultaneously cell walls are formed around each haploid nucleus, thus delimiting four haploid spores.
The spores are tetrahedrally arranged (Fig. 18 M, 20 A, F). However, in Riccia personii four spores are iso-bilaterally arranged (Khan, 1953).
The haploid number of chromosomes is 8 in species like Riccia arvensis, R. campbelliana, R. donnelleii, R. sorocarpa and R. trichocarpe. However, in species like R. austini and R. California it is 9 (Siler, 1934). In India a in polyploid species gaugetica Udar and Chopra (1957) reported 24 (n) and 48 (In) chromosomes number.
Mature Sporogonium:
A mature sporogonium is represented only by spherical spore sac or capsule. It lacks foot and seta (Fig. 19). It has a single-layered capsule wall which encloses spores. There are no elaters. A bilayered calyptra forms a protective covering around the capsule.
The capsule wall and inner layer of calyptra break down before the spore mother cells divide to form the spores. After meiosis the mass of spores lies free in the outer layer of calyptra and mature sporogonium has no diploid structure. The newly formed young gametophyte remains enclosed with in the old gametophyte (Fig. 18 M; 19).
Dispersal of Spores:
Spores are not immediately dispersed in Riccia. There is no special method of dispersal, Spores remain inside the thallus for one year or so and dispese after the death and decay of the calyptra and surrounding tissue.
The Spore:
Spores are very small (0.05-0.12 mm in diameter). They are haploid uninucleate and pyramidal in shape (Fig. 20 G). Each spore remains surrounded by three layers i.e., an outermost ornamented cutinised layer called exosporium, the middle layer called mesosporium (differentiated into 3 concentric zones) and the innermost layer called the endosporium. The endosporium is made up of cellulose and pectose (Beer, 1906).
Germination of Spores and Formation of Young Gametophyte:
Light, low temperature and water is essential for spore germination. According to Campbell (1918) the exosporium and mesosporium rupture at the triradiate mark and the endosporium comes out in the form of a tubular outgrowth called the germinal (Fig. 21 A—C).
Germinal tube is filled with cytoplasm which contains albumin granules, chloroplasts and oil granules. The germ tube elongates rapidly to form a club-shaped structure because the content of the cytoplasm move to the distal end. At the end it divides by a transverse division to form a small cell (Fig. 21 D).
It again divides by a transverse division which is parallel to first division. Both these cells divide by two vertical divisions at right angle to one another and form two tiers of four cells each. This represents octant stage (Fig. 21 E). The distal tier of four cells of the octant stage functions as an apical cell with 2 cutting faces. It cuts a number of cells on its left and right side to form the multicellular thallus (Fig. 21 G, H).
Along with these divisions the first rhizoid develops at the base of the germ Lube. Many rhizoiu develop later on from the multicellular thallus and fix it on the soil (Fig. 22 A, H).
Formation (rhizoids is affected by light intensity (Chopra and Sood, 1973). Rhizoids develop in the light i medium intensity (2000 Lux). In Riccia crustissi two spores of a tetrad develop into male thalli and two spores develop into female thalli (Mc Allister, 1928).