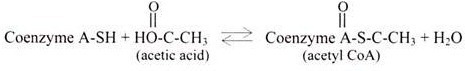This article guides you to learn about how energy is stored in Adenosine Triphosphate (ATP).
Phosphoanhydride bonds, link the terminal phosphates (formed by the removal of water between two phosphoric acids or between a carboxylic acid and a phosphoric acid) tend to have a large negative AG of hydrolysis and are thus said to be “high energy” bonds.
“High energy” bonds are often represented by the “~”symbol (squiggle), with ~P representing a phosphate group with a high free energy of hydrolysis. Compounds with high energy bonds are said to have high group transfer potential.
For example, Pi may be spontaneously removed from ATP for transfer to another compound (e.g., to a hydroxyl group on glucose). Potentially two “high energy” bonds can be cleaved from ATP, as two phosphates are released by hydrolysis from ATP (adenosine triphosphate), yielding ADP (adenosine diphosphate), and ultimately AMP (adenosine monophosphate) (Fig. 3.34).
When the third phosphate group of ATP is removed by hydrolysis, a substantial amount of free energy is released. The exact amount depends on the conditions, but generally uses a value of 7.3 kcal per mole. Thus, ATP often serves as an energy source, known as “energy currency of the cell”. Another example for molecule containing “high energy” phosphate linkage is phosphocreatine (creatine phosphate), which is used in nerve and muscle cells for storage of ~P bonds. Phosphocreatine is produced when ATP levels are high. When ATP is depleted during exercise in muscle, phosphate is transferred from phosphocreatine to ADP, to replenish ATP.
Creatine Kinase Catalyzes the Chemical Change:
Phosphocreatine + ADP □ ATP + creatine.
Phosphoenolpyruvate (PEP), involved in production of ATP in Glycolysis, has a larger negative ∆G of phosphate hydrolysis than ATP.
Removal of phosphate from the ester linkage in PEP is spontaneous because the enroll product spontaneously converts in to a ketone.
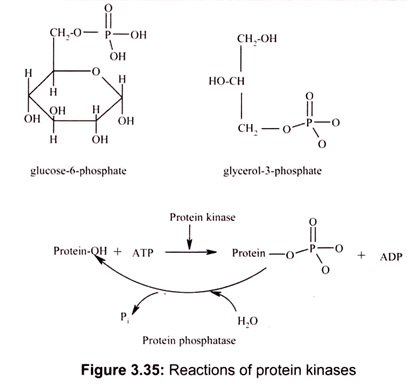
The ester linkage in PEP is an exception. Generally, phosphate esters, formed by splitting out water between a phosphoric acid and a hydroxyl group, have a low but negative ∆G of hydrolysis (Fig. 3.35). Thus, generally AMP is not further hydrolyzed in biochemical reactions for energy.
The examples of such reactions include the linkage between the first phosphate and the ribose hydroxyl of ATP, the linkage between phosphate and a hydroxyl group in glucose-6-phosphate or glycerol-3-phosphate, the linkage between phosphate and the hydroxyl group of an amino acid residue in a protein (serine, threonine or tyrosine).
ATP thus can act as a phosphate donor, and it can be synthesized by transfer of phosphate from other compounds, such as phosphoenolpyruvate (PEP).
There are several other examples for molecules containing high energy bond, viz.:
Coenzyme A:
A thioester bond is formed between a carboxylic acid and a thiol (SH) group, and are of “high energy” linkages e.g., the thiol of coenzyme A (abbreviated CoA-SH). In contrast to phosphate esters, thioesters have a large negative ∆G of hydrolysis.
The thiol of coenzyme A can react with a carboxyl group of acetic acid (yielding acetyl- CoA) or a fatty acid (yielding fatty acyl-CoA). The spontaneity of thioester cleavage is essential to the role of coenzyme A as an acyl group carrier. Like ATP, acyl-coenzyme A has a high group transfer potential.
Coenzyme A includes β-mercaptoethylamine, in amide linkage to the carboxyl group of the B vitamin pantothenate. The -OH group of pantothenate is in ester linkage to a phosphate of ADP-3′-phosphate. cAMP is an another molecule containing high energy bond.