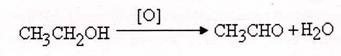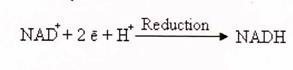After reading this article you will learn about the oxidation and reduction reactions of substances.
A chemical reaction can be defined as a change in which a substance (or substances) is changed into one or more new substances. In redox reactions both reduction and the oxidation processes go on side-by-side.
The oxidation and reduction reactions can be explained in three different ways:
(a) On the basis of oxygen transfer:
Oxidation is a gain of oxygen while reduction is loss of oxygen.
For example:
(b) In terms of hydrogen transfer:
Oxidation is loss of hydrogen and reduction is gain of hydrogen.
For example, ethanol can be oxidized to ethyl acetate,
(c) In terms of electron transfer:
Oxidation is loss of electrons and reduction is gain of electrons.
For example:
Reaction of Nicotinamide Adenine Dinucleotide (NAD):
Nicotinamide Adenine Dinucleotide functions as an electron acceptor in catabolic pathways. The nicotinamide ring of NAD+, which is derived from the vitamin niacin, accepts 2 e– and one H+ (a hydride) in going to the reduced state, as NAD+ becomes NADH. NADP+ / NADPH is similar, except for an additional phosphate esterified to a hydroxyl group on the adenosine ribose. NADPH functions as an electron donor in synthetic pathways. NAD+ is a coenzyme that reversibly binds to enzymes (Fig. 3.36).
The electron transfer reaction may be summarized as:
NAD+ + 2 é + H+ –> NADH also be written as NAD+ + 2 é + 2H+ –> NADH + H+
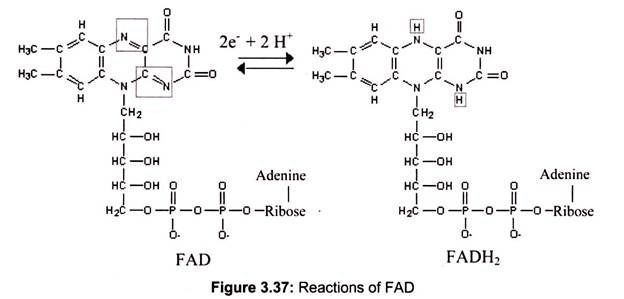
Reactions of Flavin Adenine Dinucleotide (FAD):
Flavin Adenine Dinucleotide also functions as an electron acceptor. The portion of FAD that undergoes reduction/oxidation is the dimethylisoalloxazine ring, derived from the vitamin riboflavin. FAD is a prosthetic group that usually remains tightly bound at the active site of an enzyme.
FAD normally accepts 2 é and 2 H+ in going to its reduced state (Fig.3.37):
FAD + 2 é + 2 H+ —> FADH2