The following points highlight the three main types of growth that take place in bacteria. The types are: 1. Diauxic Growth 2. Synchronous Growth 3. Continuous Growth.
Type # 1. Diauxic Growth (Diphasic Growth):
Diauxic growth is a diphasic growth represented by two growth curves intervened by a short lag phase produced by an organism utilizing two different substrates, one of which is glucose. When E. coli grows in a medium containing both glucose and lactose, it uses glucose preferentially until the glucose is exhausted.
Then after a short lag phase during which bacterium synthesizes the enzymes needed for lactose use, growth resumes with lactose as a carbon source. If this diphasic growth of E. coli is plotted in respect to bacterial density against time, two growth curves follow one after the other intervened by a short lag phase to produce a diauxic or diphasic growth curve (Fig. 19.3).
The enzyme needed for lactose use is β-galactosidase, which splits lactose into glucose and galactose, and the bacterium utilizes glucose for growth. Galactose can also be utilized, but only after it is converted to glucose. It has been demonstrated that E. coli growing in a medium containing both glucose and galactose produces a diauxic (diphasic) growth curve as in case of glucose and lactose.
Similar response has been found in case of other sugars such as arabinose, maltose, sorbitol, etc. when they are used in combination with glucose by E. coli. Each of these sugars is utilized only after glucose has been used up in the growth medium.
The cause of diauxic (diphasic) growth is complex and not completely understood, it is considered that catabolite repression or the glucose effect probably plays a part in it. In catabolite repression of the lac-operon of E. coli, glucose exerts an inhibitory effect on the transcription of the lac genes.
As a result, lactose- utilization enzymes are not synthesized, even if lactose is present in the medium. When glucose is completely consumed by E. coli, the bacterium is now competent to transcribe the lac-operon genes resulting in production of necessary enzymes that help metabolise lactose.
Type # 2. Synchronous Growth:
Synchronous growth of a bacterial population is that during which all bacterial cells of the population are physiologically identical and in the same stage of cell division cycle at a given time. Synchronous growth helps studying particular stages or the cell division cycle and their interrelations.
In most of the bacterial cultures the stages of growth and cell division cycle are completely random and thus it becomes difficult to understand the properties during the course of division cycle using such cultures. To overcome this problem, the microbiologists have developed synchronous culture techniques to find synchronous growth of bacterial population.
Synchronous culture is that in which the growth is synchronous i.e. all the bacterial cells of the population are physiologically identical and in the same stage of cell division cycle at a given time.
A synchronous culture can be obtained either by manipulating environmental conditions such as by repeatedly changing the temperature or by adding fresh nutrients to cultures as soon as they enter the stationary phase, or by physical separation of cells by centrifugation or filtration.
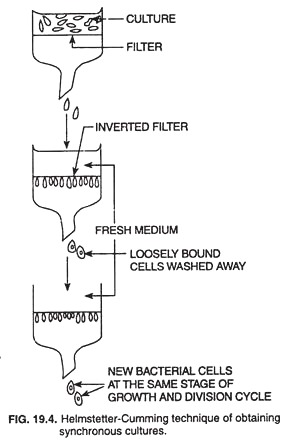
An excellent and most widely used method to obtain synchronous cultures is the Helmstetter-Cummings Technique (Fig. 19.4) in which an unsynchronized bacterial culture is filtered through cellulose nitrate membrane filter.
The loosely bound bacterial cells are washed from the filter, leaving some cells tightly associated with the filter. The filter is now inverted and fresh medium is allowed to flow through it.
New bacterial cells, that are produced by cell division and are not lightly associated with the filter, are washed into the effluent. Hence, all cells in the effluent are newly formed and are, therefore at the same stage of growth and division cycle. The effluent thus represents a synchronous culture.
Type # 3. Continuous Growth: Chemo- Stat and Turbidostat:
Contrary to the studies in batch culture where the exponential growth of bacterial population is restricted only for a few generations, it is often desirable to maintain prolonged exponential growth of bacterial population for genetical and biochemical studies, and in industrial processes.
This condition is obtained by growing bacteria in a continuous culture, a culture in which nutrients arc supplied and end products continuously removed.
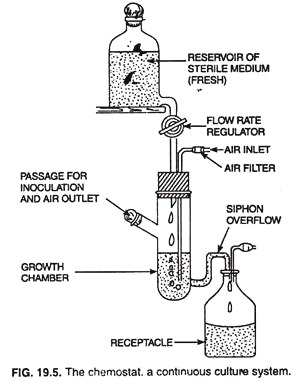
A continuous culture, therefore, is that in which the exponential growth phase of bacterial population can be maintained at a constant rate (steady state growth) for over a long period of time by continuously supplying fresh medium from a reservoir to growth chamber and continuously removing excess volume of culture medium of growth chamber through a siphon overflow.
By doing so the microbes never reach stationary phase because the end products do not accumulate to work as inhibitory to growth and nutrients arc not completely expended.
Continuous culture systems can be operated as chemostats or as turbidostats. In a chemostat (Fig. 19.5) the flow rate is set at a particular value with the help of a flow rate regulator and the rate of growth of the culture adjusts to this flow rate. That is, the sterile medium is fed into the vessel at the same rate as the media containing microorganisms is removed.
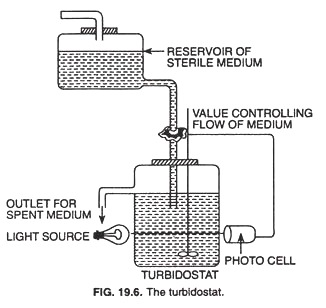
In a turbidostat (Fig. 19.6), the system includes an optical sensing device (photoelectric device) which continuously monitors the culture density in the growth vessel and controls the dilution rate to maintain the culture density at a constant rate. If the culture density becomes too high the dilution rate is increased, and if it becomes too low the dilution rate is decreased.
The turbidostat differs from the chemostat in many ways. The dilution rate in a turbidostat varies rather than remaining constant, and its culture medium lacks a limiting nutrient. The turbidostat operates best at high dilution rates; the chemostat is most stable and effective at low dilution rates.